Daily Rhythms of TNFα Expression and Food Intake Regulate Synchrony of Plasmodium Stages with the Host Circadian Cycle.
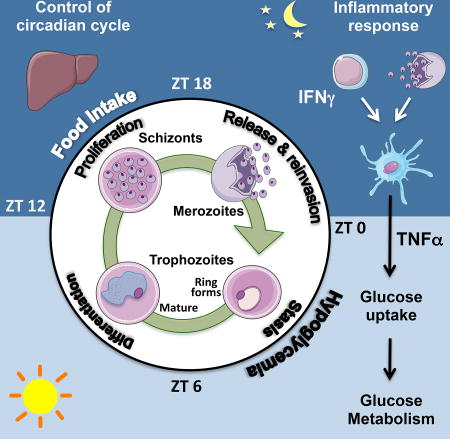
The Plasmodium cell cycle, wherein millions of parasites differentiate and proliferate, occurs in synchrony with the vertebrate host's circadian cycle. The underlying mechanisms are unknown. Here we addressed this question in a mouse model of Plasmodium chabaudi infection. Inflammatory gene expression and carbohydrate metabolism are both enhanced in interferon-γ (IFNγ)-primed leukocytes and liver cells from P. chabaudi-infected mice. Tumor necrosis factor α (TNFα) expression oscillates across the host circadian cycle, and increased TNFα correlates with hypoglycemia and a higher frequency of non-replicative ring forms of trophozoites. Conversely, parasites proliferate and acquire biomass during food intake by the host. Importantly, cyclic hypoglycemia is attenuated and synchronization of P. chabaudi stages is disrupted in IFNγ -/- , TNF receptor -/- , or diabetic mice. Hence, the daily rhythm of systemic TNFα production and host food intake set the pace for Plasmodium synchronization with the host's circadian cycle. This mechanism indicates that Plasmodium parasites take advantage of the host's feeding habits.
Authors
Isabella Cristina Hirako; Patrícia Aparecida Assis; Natália Satchiko Hojo-Souza; George Reed; Helder Nakaya; Douglas Taylor Golenbock; Roney Santos Coimbra; Ricardo Tostes Gazzinelli
External link
Publication Year
Publication Journal
Associeted Project
Systems Immunology of Human Diseases
Lista de serviços
-
Is the gut microbiome key to modulating vaccine efficacy?Is the gut microbiome key to modulating vaccine efficacy?
-
Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.