Profiling plasma-extracellular vesicle proteins and microRNAs in diabetes onset in middle-aged male participants in the ELSA-Brasil study.
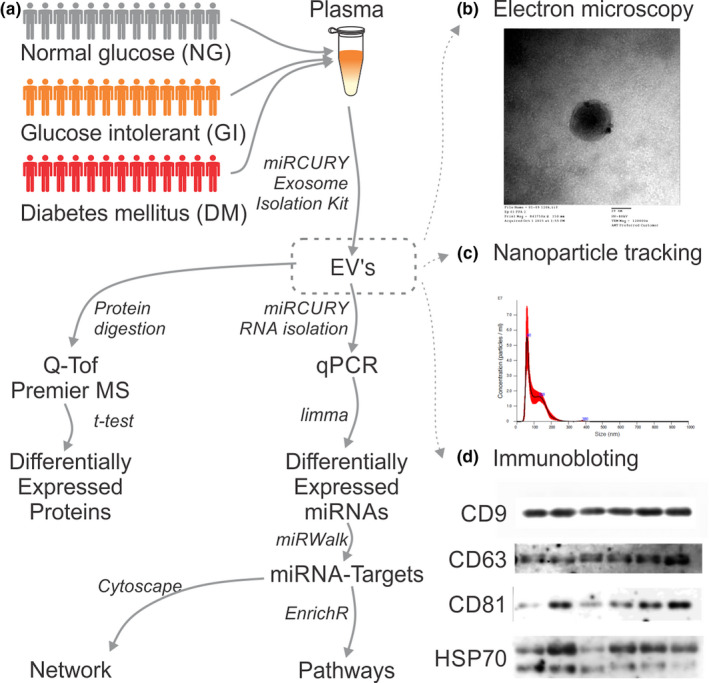
We measured plasma-derived extracellular vesicle (EV) proteins and their microRNA (miRNA) cargos in normoglycemic (NG), glucose intolerant (GI), and newly diagnosed diabetes mellitus (DM) in middle-aged male participants of the Brazilian Longitudinal Study of Adult Health (ELSA-Brazil). Mass spectrometry revealed decreased IGHG-1 and increased ITIH2 protein levels in the GI group compared with that in the NG group and higher serotransferrin in EVs in the DM group than in those in the NG and GI groups. The GI group also showed increased serum ferritin levels, as evaluated by biochemical analysis, compared with those in both groups. Seventeen miRNAs were differentially expressed (DEMiRs) in the plasma EVs of the three groups. DM patients showed upregulation of miR-141-3p and downregulation of miR-324-5p and -376c-3p compared with the NG and GI groups. The DM and GI groups showed increased miR-26b-5p expression compared with that in the NG group. The DM group showed decreased miR-374b-5p levels compared with those in the GI group and higher concentrations than those in the NG group. Thus, three EV proteins and five DEMiR cargos have potential prognostic importance for diabetic complications mainly associated with the immune function and iron status of GI and DM patients.
Authors
Laureane N Masi; Paulo A Lotufo; Frederico M Ferreira; Alice C Rodrigues; Tamires D A Serdan; Talita Souza-Siqueira; Aécio A Braga; Magda E G Saldarriaga; Tatiana C Alba-Loureiro; Fernanda T Borges; Diego P Cury; Mario H Hirata; Renata Gorjão; Tania C Pithon-Curi; Simão A Lottenberg; Ligia M G Fedeli; Helder T I Nakaya; Isabela J M Bensenor; Rui Curi; Sandro M Hirabara
External link
Publication Year
Publication Journal
Associeted Project
Integrative Biology
Lista de serviços
-
Is the gut microbiome key to modulating vaccine efficacy?Is the gut microbiome key to modulating vaccine efficacy?
-
Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.