Activation of the IDO1-GCN2-ATF4-CHOP Pathway During the Massive Generation of Antibody-Secreting Cells in Dengue Patients Through Single-Cell Transcriptomics.
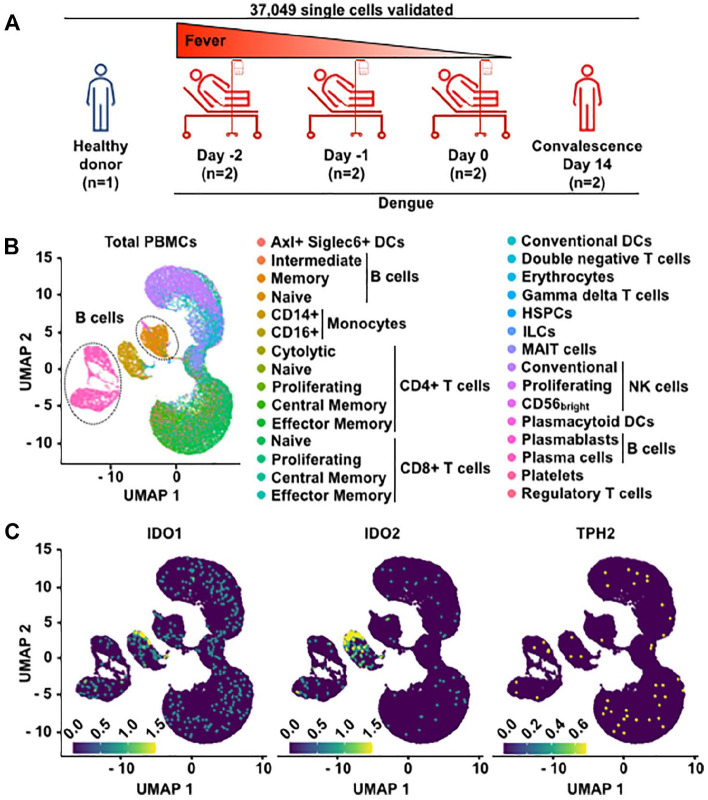
Dengue, a widespread mosquito-borne disease, annually afflicts millions globally, posing substantial mortality risks. Preceding disease defervescence, a marked and transient surge in antibody-secreting cell (ASC) frequency correlates with disease severity, paralleled by heightened tryptophan degradation. Investigating details of this process through single-cell transcriptomics from public repositories, our data pinpoint CD14+ monocytes as principal IDO1 and IDO2 expressors, implicating them, rather than B cells, in initiating tryptophan metabolism. Interestingly, naive B cells exhibit altered gene expression indicative of early impact by tryptophan deficiency before defervescence with a potential impact on the B cell fate. Dengue-induced ASCs upregulated GCN2, PERK, eIF2a, ATF4 genes as well as BIM and CASP-3. However, the high expression of anti-apoptotic genes (FKBP8 [a CHOP-regulated gene], BCL-XL, BCL-2, MCL-1) allows enhanced ASC survival. Proliferation and differentiation-related genes (eIF4EBP1, RRM2, and HIF1a) were also upregulated in ASCs. These findings untangle how Dengue modulates the host metabolism and B-cell responses, although further research is needed to fully understand their implications on disease progression.
Authors
Nascimento JC, Gonçalves ANA, Akashi KT, Nakaya HI, et al.
External link
Publication Year
Publication Journal
Associeted Project
Systems Immunology of Human Diseases
Lista de serviços
-
Is the gut microbiome key to modulating vaccine efficacy?Is the gut microbiome key to modulating vaccine efficacy?
-
Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.