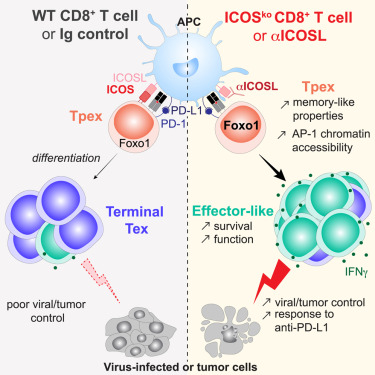
The costimulatory molecule ICOS limits memory-like properties and function of exhausted PD-1(+)CD8(+) T cells.
During persistent antigen stimulation, CD8+ T cell responses are maintained by progenitor exhausted CD8+ T (Tpex) cells. Tpex cells respond to blockade of the inhibitory receptor programmed cell death-1 (PD-1), and regulation of their differentiation is critical for immunotherapies. Tpex cells highly express inducible costimulator (ICOS), but how ICOS modulates PD-1+CD8+ T cells is not clear. During chronic infection, intrinsic ICOS deficiency increased number and quality of virus-specific CD8+ T cells. Loss of ICOS potentiated activity of the transcription factor forkhead box O1 (FoxO1) and memory-like features of Tpex cells. ICOS-deficient Tpex cells were poised to generate effecor-like cells with improved survival and cytokine production. ICOS-ligand (ICOSL) blockade expanded effector-like PD-1+CD8+ T cells, reduced viral load, and improved response to PD-1 blockade. Similarly, in a mouse model of hepatocellular carcinoma, ICOS inhibition enhanced tumor-specific CD8+ T cell responses and tumor control by PD-1 blockade. Overall, we show that sustained ICOS costimulation limits CD8+ T cell responses during chronic antigen exposure.
Authors
Humblin E, Korpas I, Prokhnevska N, Vaidya A, Filipescu D, et al.
External link
Publication Year
Publication Journal
Associeted Project
Systems Immunology of Human Diseases
Lista de serviços
-
Is the gut microbiome key to modulating vaccine efficacy?Is the gut microbiome key to modulating vaccine efficacy?
-
Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.Toxicogenomic and bioinformatics platforms to identify key molecular mechanisms of a curcumin-analogue DM-1 toxicity in melanoma cells.