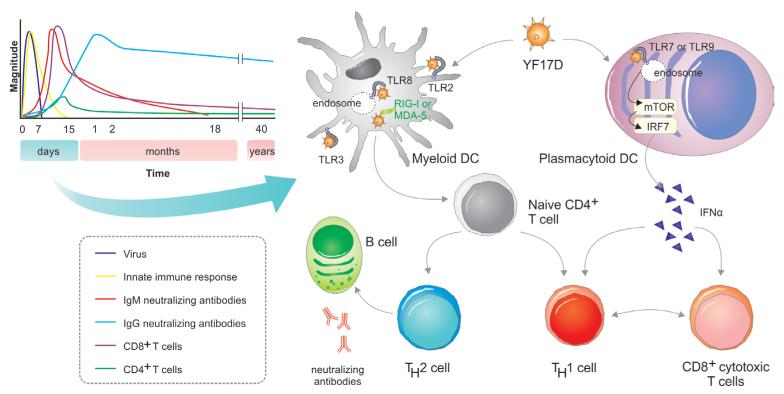
Immunity to viruses: learning from successful human vaccines.
For more than a century, immunologists and vaccinologists have existed in parallel universes. Immunologists have for long reveled in using 'model antigens', such as chicken egg ovalbumin or nitrophenyl haptens, to study immune responses in model organisms such as mice. Such studies have yielded many seminal insights about the mechanisms of immune regulation, but their relevance to humans has been questioned. In another universe, vaccinologists have relied on human clinical trials to assess vaccine efficacy, but have done little to take advantage of such trials for studying the nature of immune responses to vaccination. The human model provides a nexus between these two universes, and recent studies have begun to use this model to study the molecular profile of innate and adaptive responses to vaccination. Such 'systems vaccinology' studies are beginning to provide mechanistic insights about innate and adaptive immunity in humans. Here, we present an overview of such studies, with particular examples from studies with the yellow fever and the seasonal influenza vaccines. Vaccination with the yellow fever vaccine causes a systemic acute viral infection and thus provides an attractive model to study innate and adaptive responses to a primary viral challenge. Vaccination with the live attenuated influenza vaccine causes a localized acute viral infection in mucosal tissues and induces a recall response, since most vaccinees have had prior exposure to influenza, and thus provides a unique opportunity to study innate and antigen-specific memory responses in mucosal tissues and in the blood. Vaccination with the inactivated influenza vaccine offers a model to study immune responses to an inactivated immunogen. Studies with these and other vaccines are beginning to reunite the estranged fields of immunology and vaccinology, yielding unexpected insights about mechanisms of viral immunity. Vaccines that have been proven to be of immense benefit in saving lives offer us a new fringe benefit: lessons in viral immunology.
Authors
Bali Pulendran; Jason Z Oh; Helder I Nakaya; Rajesh Ravindran; Dmitri A Kazmin
External link
Publication Year
Publication Journal
Associeted Project
Systems Vaccinology
Lista de serviços
-
RASL11A, member of a novel small monomeric GTPase gene family, is down-regulated in prostate tumors.RASL11A, member of a novel small monomeric GTPase gene family, is down-regulated in prostate tumors.
-
Splice variants of TLE family genes and up-regulation of a TLE3 isoform in prostate tumors.Splice variants of TLE family genes and up-regulation of a TLE3 isoform in prostate tumors.
-
Concepts on Microarray Design for Genome and Transcriptome AnalysesConcepts on Microarray Design for Genome and Transcriptome Analyses
-
The iron stimulon of Xylella fastidiosa includes genes for type IV pilus and colicin V-like bacteriocins.The iron stimulon of Xylella fastidiosa includes genes for type IV pilus and colicin V-like bacteriocins.
-
Origins of the Xylella fastidiosa prophage-like regions and their impact in genome differentiation.Origins of the Xylella fastidiosa prophage-like regions and their impact in genome differentiation.
-
The role of prophage in plant-pathogenic bacteria.The role of prophage in plant-pathogenic bacteria.
-
Genetic control of immune response and susceptibility to infectious diseases.Genetic control of immune response and susceptibility to infectious diseases.
-
Building capacity for advances in tuberculosis research; proceedings of the third RePORT international meeting.Building capacity for advances in tuberculosis research; proceedings of the third RePORT international meeting.
-
São Paulo School of Advanced Sciences on Vaccines: an overview.São Paulo School of Advanced Sciences on Vaccines: an overview.
-
A reasonable request for true data sharing.A reasonable request for true data sharing.