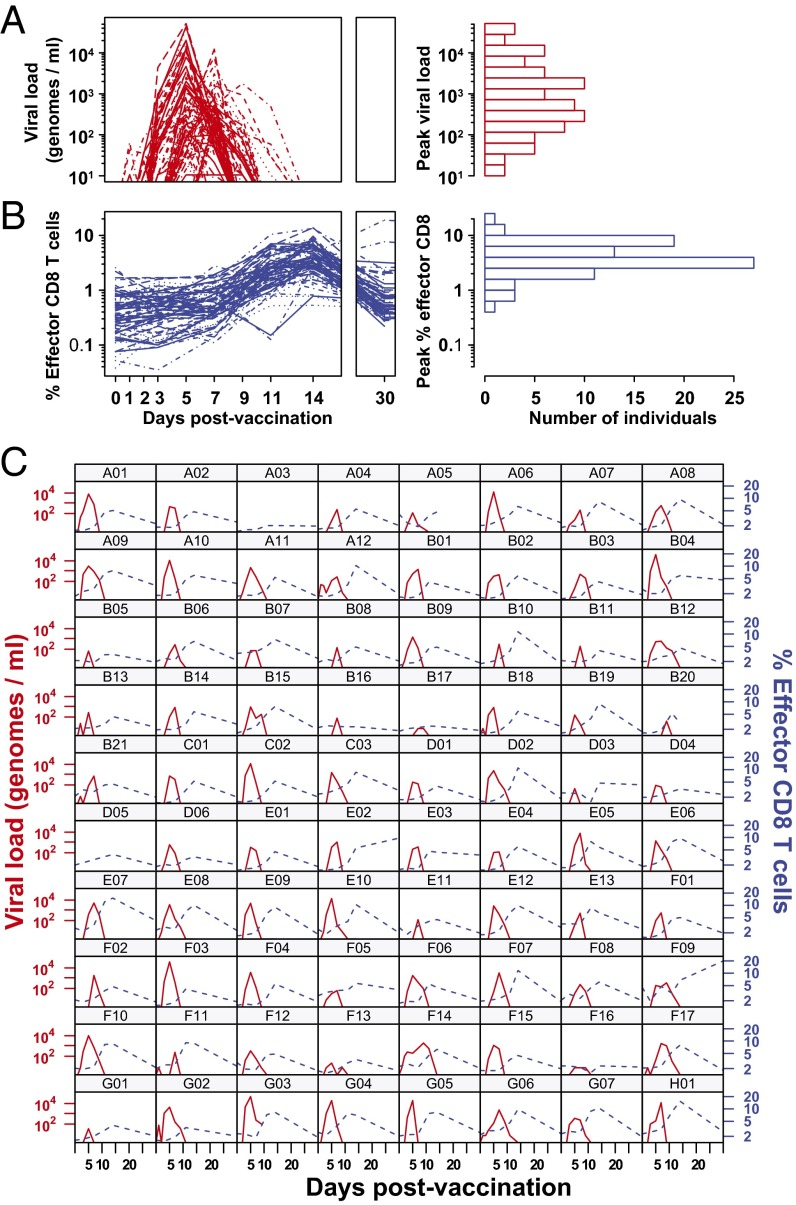
Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination.
CD8 T cells are a potent tool for eliminating intracellular pathogens and tumor cells. Thus, eliciting robust CD8 T-cell immunity is the basis for many vaccines under development. However, the relationship between antigen load and the magnitude of the CD8 T-cell response is not well-described in a human immune response. Here we address this issue by quantifying viral load and the CD8 T-cell response in a cohort of 80 individuals immunized with the live attenuated yellow fever vaccine (YFV-17D) by sampling peripheral blood at days 0, 1, 2, 3, 5, 7, 9, 11, 14, 30, and 90. When the virus load was below a threshold (peak virus load < 225 genomes per mL, or integrated virus load < 400 genome days per mL), the magnitude of the CD8 T-cell response correlated strongly with the virus load (R(2) ∼ 0.63). As the virus load increased above this threshold, the magnitude of the CD8 T-cell responses saturated. Recent advances in CD8 T-cell-based vaccines have focused on replication-incompetent or single-cycle vectors. However, these approaches deliver relatively limited amounts of antigen after immunization. Our results highlight the requirement that T-cell-based vaccines should deliver sufficient antigen during the initial period of the immune response to elicit a large number of CD8 T cells that may be needed for protection.
Authors
Rama S Akondy; Philip L F Johnson; Helder I Nakaya; Srilatha Edupuganti; Mark J Mulligan; Benton Lawson; Joseph D Miller; Bali Pulendran; Rustom Antia; Rafi Ahmed
External link
Publication Year
Publication Journal
Associeted Project
Microbiology or Immunology
Lista de serviços
-
As antisense RNA gets intronic.As antisense RNA gets intronic.
-
Androgen responsive intronic non-coding RNAs.Androgen responsive intronic non-coding RNAs.
-
Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.
-
The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.
-
Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.
-
Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.
-
Long non-coding RNAs associated with infection and vaccine-induced immunityLong non-coding RNAs associated with infection and vaccine-induced immunity
-
Comparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophagesComparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophages
-
SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.