Uptake of Plasmodium chabaudi hemozoin drives Kupffer cell death and fuels superinfections
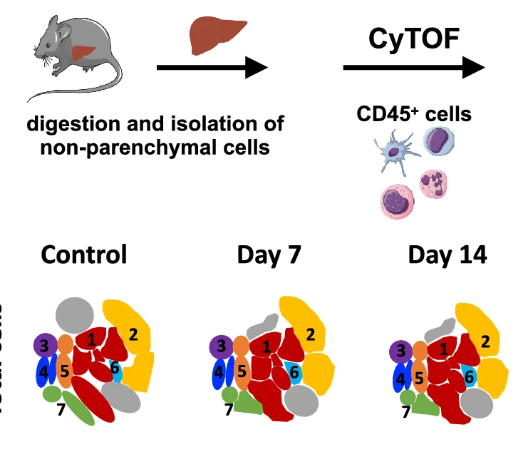
Kupffer cells (KCs) are self-maintained tissue-resident macrophages that line liver sinusoids and play an important role on host defense. It has been demonstrated that upon infection or intense liver inflammation, KCs might be severely depleted and replaced by immature monocytic cells; however, the mechanisms of cell death and the alterations on liver immunity against infections deserves further investigation. We explored the impact of acute Plasmodium infection on KC biology and on the hepatic immune response against secondary infections. Similar to patients, infection with Plasmodium chabaudi induced acute liver damage as determined by serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevation. This was associated with accumulation of hemozoin, increased of proinflammatory response and impaired bacterial and viral clearance, which led to pathogen spread to other organs. In line with this, mice infected with Plasmodium had enhanced mortality during secondary infections, which was associated with increased production of mitochondrial superoxide, lipid peroxidation and increased free iron within KCs—hallmarks of cell death by ferroptosis. Therefore, we revealed that accumulation of iron with KCs, triggered by uptake of circulating hemozoin, is a novel mechanism of macrophage depletion and liver inflammation during malaria, providing novel insights on host susceptibility to secondary infections. Malaria can cause severe liver damage, along with depletion of liver macrophages, which can predispose individuals to secondary infections and enhance the chances of death.
Authors
Hirako, Isabella C; Antunes, Maisa Mota; Rezende, Rafael Machado; Hojo-Souza, Nati¡lia Satchiko; Figueiredo, Maria Marta; Dias, Thomaz; Nakaya, Helder; Menezes, Gustavo Batista; Gazzinelli, Ricardo Tostes;
External link
Publication Year
Publication Journal
Associeted Project
Microbiology or Immunology
Lista de serviços
-
As antisense RNA gets intronic.As antisense RNA gets intronic.
-
Androgen responsive intronic non-coding RNAs.Androgen responsive intronic non-coding RNAs.
-
Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.
-
The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.
-
Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.
-
Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.
-
Long non-coding RNAs associated with infection and vaccine-induced immunityLong non-coding RNAs associated with infection and vaccine-induced immunity
-
Comparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophagesComparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophages
-
SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.