Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.
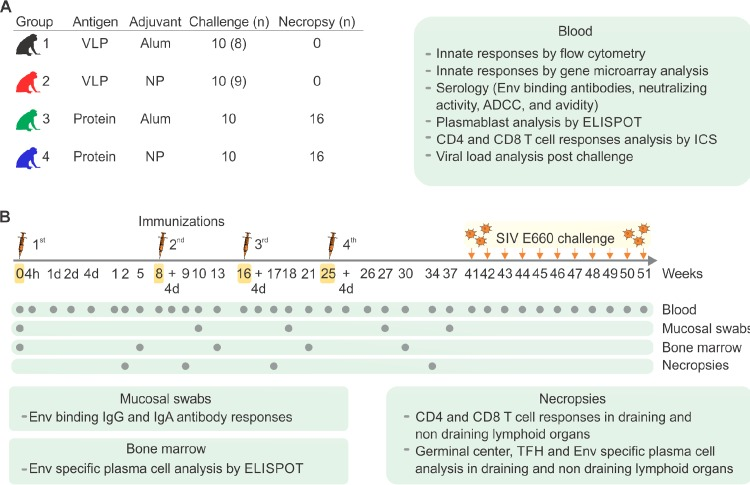
Our previous work has shown that antigens adjuvanted with ligands specific for Toll-like receptor 4 (TLR4) and TLR7/8 encapsulated in poly(lactic-co-glycolic) acid (PLGA)-based nanoparticles (NPs) induce robust and durable immune responses in mice and macaques. We investigated the efficacy of these NP adjuvants in inducing protective immunity against simian immunodeficiency virus (SIV). Rhesus macaques (RMs) were immunized with NPs containing TLR4 and TLR7/8 agonists mixed with soluble recombinant SIVmac239-derived envelope (Env) gp140 and Gag p55 (protein) or with virus-like particles (VLPs) containing SIVmac239 Env and Gag. NP-adjuvanted vaccines induced robust innate responses, antigen-specific antibody responses of a greater magnitude and persistence, and enhanced plasmablast responses compared to those achieved with alum-adjuvanted vaccines. NP-adjuvanted vaccines induced antigen-specific, long-lived plasma cells (LLPCs), which persisted in the bone marrow for several months after vaccination. NP-adjuvanted vaccines induced immune responses that were associated with enhanced protection against repeated low-dose, intravaginal challenges with heterologous SIVsmE660 in animals that carried TRIM5α restrictive alleles. The protection induced by immunization with protein-NP correlated with the prechallenge titers of Env-specific IgG antibodies in serum and vaginal secretions. However, no such correlate was apparent for immunization with VLP-NP or alum as the adjuvant. Transcriptional profiling of peripheral blood mononuclear cells isolated within the first few hours to days after primary vaccination revealed that NP-adjuvanted vaccines induced a molecular signature similar to that induced by the live attenuated yellow fever viral vaccine. This systems approach identified early blood transcriptional signatures that correlate with Env-specific antibody responses in vaginal secretions and protection against infection. These results demonstrate the adjuvanticity of the NP adjuvant in inducing persistent and protective antibody responses against SIV in RMs with implications for the design of vaccines against human immunodeficiency virus (HIV). The results of the RV144 HIV vaccine trial, which demonstrated a rapid waning of protective immunity with time, have underscored the need to develop strategies to enhance the durability of protective immune responses. Our recent work in mice has highlighted the capacity of nanoparticle-encapsulated TLR ligands (NP) to induce potent and durable antibody responses that last a lifetime in mice. In the present study, we evaluated the ability of these NP adjuvants to promote robust and durable protective immune responses against SIV in nonhuman primates. Our results demonstrate that immunization of rhesus macaques with NP adjuvants mixed with soluble SIV Env or a virus-like particle form of Env (VLP) induces potent and durable Env-specific antibody responses in the serum and in vaginal secretions. These responses were superior to those induced by alum adjuvant, and they resulted in enhanced protection against a low-dose intravaginal challenge with a heterologous strain of SIV in animals with TRIM5a restrictive alleles. These results highlight the potential for such NP TLR L adjuvants in promoting robust and durable antibody responses against HIV in the next generation of HIV immunogens currently being developed.
Authors
Sudhir Pai Kasturi; Pamela A Kozlowski; Helder I Nakaya; Matheus C Burger; Pedro Russo; Mathew Pham; Yevgeniy Kovalenkov; Eduardo L V Silveira; Colin Havenar-Daughton; Samantha L Burton; Katie M Kilgore; Mathew J Johnson; Rafiq Nabi; Traci Legere; Zarpheen Jinnah Sher; Xuemin Chen; Rama R Amara; Eric Hunter; Steven E Bosinger; Paul Spearman; Shane Crotty; Francois Villinger; Cynthia A Derdeyn; Jens Wrammert; Bali Pulendran
External link
Publication Year
Publication Journal
Associeted Project
Systems Vaccinology
Lista de serviços
-
Systems vaccinology: its promise and challenge for HIV vaccine development.Systems vaccinology: its promise and challenge for HIV vaccine development.
-
Systems vaccinology: learning to compute the behavior of vaccine induced immunity.Systems vaccinology: learning to compute the behavior of vaccine induced immunity.
-
Systems biology of vaccination in the elderly.Systems biology of vaccination in the elderly.
-
Immunity to viruses: learning from successful human vaccines.Immunity to viruses: learning from successful human vaccines.
-
Systems biological approaches to measure and understand vaccine immunity in humans.Systems biological approaches to measure and understand vaccine immunity in humans.
-
Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response.Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response.
-
Vaccinology in the era of high-throughput biology.Vaccinology in the era of high-throughput biology.
-
Systems vaccinology: Enabling rational vaccine design with systems biological approaches.Systems vaccinology: Enabling rational vaccine design with systems biological approaches.
-
Systems Vaccinology Applied to DNA Vaccines: Perspective and Challenges.Systems Vaccinology Applied to DNA Vaccines: Perspective and Challenges.
-
Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.
-
Methods for predicting vaccine immunogenicity and reactogenicity.Methods for predicting vaccine immunogenicity and reactogenicity.
-
Antigenicity prediction and vaccine recommendation of human influenza virus A (H3N2) using convolutional neural networks.Antigenicity prediction and vaccine recommendation of human influenza virus A (H3N2) using convolutional neural networks.
-
Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine.Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine.
-
Human Transcriptomic Response to the VSV-Vectored Ebola Vaccine.Human Transcriptomic Response to the VSV-Vectored Ebola Vaccine.
-
Induction of Cell Cycle and NK Cell Responses by Live-Attenuated Oral Vaccines against Typhoid Fever.Induction of Cell Cycle and NK Cell Responses by Live-Attenuated Oral Vaccines against Typhoid Fever.
-
Systems Biology Analysis of the Radiation-Attenuated Schistosome Vaccine Reveals a Role for Growth Factors in Protection and Hemostasis Inhibition in Parasite Survival.Systems Biology Analysis of the Radiation-Attenuated Schistosome Vaccine Reveals a Role for Growth Factors in Protection and Hemostasis Inhibition in Parasite Survival.
-
Molecular alterations in human milk in simulated maternal nasal mucosal infection with live attenuated influenza vaccinationMolecular alterations in human milk in simulated maternal nasal mucosal infection with live attenuated influenza vaccination
-
Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood.Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood.
-
Systems analysis of protective immune responses to RTS,S malaria vaccination in humans.Systems analysis of protective immune responses to RTS,S malaria vaccination in humans.
-
Systems vaccinology.Systems vaccinology.
-
Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans.Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans.
-
Systems biology of vaccination for seasonal influenza in humans.Systems biology of vaccination for seasonal influenza in humans.
-
Prior upregulation of interferon pathways in the nasopharynx impacts viral shedding following live attenuated influenza vaccine challenge in childrenPrior upregulation of interferon pathways in the nasopharynx impacts viral shedding following live attenuated influenza vaccine challenge in children
-
Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures.Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures.
-
Molecular signatures of antibody responses derived from a systems biology study of five human vaccines.Molecular signatures of antibody responses derived from a systems biology study of five human vaccines.
-
Long noncoding RNAs are involved in multiple immunological pathways in response to vaccination.Long noncoding RNAs are involved in multiple immunological pathways in response to vaccination.
-
Metabolic Phenotypes of Response to Vaccination in Humans.Metabolic Phenotypes of Response to Vaccination in Humans.
-
Hidden in plain sight: uncovering the role of CREB1 in HIV-1 vaccine-induced immunityHidden in plain sight: uncovering the role of CREB1 in HIV-1 vaccine-induced immunity
-
Transcriptomic signatures induced by the Ebola virus vaccine rVSV-ZEBOV-GP in adult cohorts in Europe, Africa, and North America: a molecular biomarker studyTranscriptomic signatures induced by the Ebola virus vaccine rVSV-ZEBOV-GP in adult cohorts in Europe, Africa, and North America: a molecular biomarker study
-
Baseline gene signatures of reactogenicity to Ebola vaccination: a machine learning approach across multiple cohortsBaseline gene signatures of reactogenicity to Ebola vaccination: a machine learning approach across multiple cohorts
-
Global blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GPGlobal blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GP
-
COVID-19 vaccination atlas using an integrative systems vaccinology approach.COVID-19 vaccination atlas using an integrative systems vaccinology approach.
-
Emulsion adjuvant-induced uric acid release modulates optimal immunogenicity by targeting dendritic cells and B cells.Emulsion adjuvant-induced uric acid release modulates optimal immunogenicity by targeting dendritic cells and B cells.
-
System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans.System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans.
-
Poly I:C elicits broader and stronger humoral and cellular responses to a Plasmodium vivax circumsporozoite protein malaria vaccine than Alhydrogel in mice.Poly I:C elicits broader and stronger humoral and cellular responses to a Plasmodium vivax circumsporozoite protein malaria vaccine than Alhydrogel in mice.