Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study.
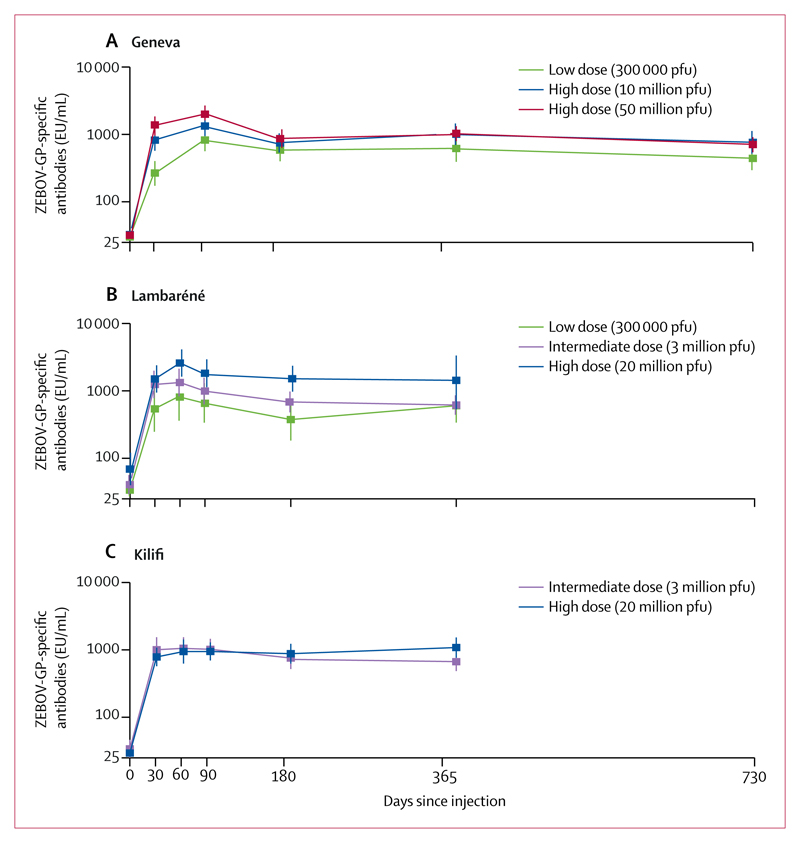
The recombinant vesicular stomatitis virus (rVSV) vaccine expressing the Zaire Ebola virus (ZEBOV) glycoprotein is efficacious in the weeks following single-dose injection, but duration of immunity is unknown. We aimed to assess antibody persistence at 1 and 2 years in volunteers who received single-dose rVSV-ZEBOV in three previous trials. In this observational cohort study, we prospectively followed-up participants from the African and European phase 1 rVSV-ZEBOV trials, who were vaccinated once in 2014-15 with 300 000 (low dose) or 10-50 million (high dose) plaque-forming units (pfu) of rVSV-ZEBOV vaccine to assess ZEBOV glycoprotein (IgG) antibody persistence. The primary outcome was ZEBOV glycoprotein-specific IgG geometric mean concentrations (GMCs) measured yearly by ELISA compared with 1 month (ie, 28 days) after immunisation. We report GMCs up to 2 years (Geneva, Switzerland, including neutralising antibodies up to 6 months) and 1 year (Lambaréné, Gabon; Kilifi, Kenya) after vaccination and factors associated with higher antibody persistence beyond 6 months, according to multivariable analyses. Trials and the observational study were registered at ClinicalTrials.gov (Geneva: NCT02287480 and NCT02933931; Kilifi: NCT02296983) and the Pan-African Clinical Trials Registry (Lambaréné PACTR201411000919191). Of 217 vaccinees from the original studies (102 from the Geneva study, 75 from the Lambaréné study, and 40 from the Kilifi study), 197 returned and provided samples at 1 year (95 from the Geneva study, 63 from the Lambaréné, and 39 from the Kilifi study) and 90 at 2 years (all from the Geneva study). In the Geneva group, 44 (100%) of 44 participants who had been given a high dose (ie, 10-50 million pfu) of vaccine and who were seropositive at day 28 remained seropositive at 2 years, whereas 33 (89%) of 37 who had been given the low dose (ie, 300 000 pfu) remained seropositive for 2 years (p=0·042). In participants who had received a high dose, ZEBOV glycoprotein IgG GMCs decreased significantly between their peak (at 1-3 months) and month 6 after vaccination in Geneva (p<0·0001) and Lambaréné (p=0·0298) but not in Kilifi (p=0·5833) and subsequently remained stable at all sites apart from Geneva, where GMC in those given a high dose of vaccine increased significantly between 6 months and 1 year (p=0·0264). Antibody persistence was similar at 1 year and at 6 months in those who had received a low dose of vaccine, with lower titres among participants from the Geneva study at 2 years than at 1 year after vaccination (GMC ratio 0·61, 95% CI 0·49-0·77; p<0·0001). In multivariable analyses, predictors of increased IgG GMCs beyond 6 months included high-dose versus low-dose vaccination (Geneva p=0·0133; Lambaréné p=0·008) and vaccine-related arthritis (p=0·0176), but not sex, age, or baseline seropositivity (all p>0·05). Neutralising antibodies seem to be less durable, with seropositivity dropping from 64-71% at 28 days to 27-31% at 6 months in participants from the Geneva study. Antibody responses to single-dose rVSV-ZEBOV vaccination are sustained across dose ranges and settings, a key criterion in countries where booster vaccinations would be impractical. The Wellcome Trust and Innovative Medicines Initiative 2 Joint Undertaking.
Authors
Angela Huttner; Selidji Todagbe Agnandji; Christophe Combescure; José F Fernandes; Emmanuel Bache Bache; Lumeka Kabwende; Francis Maina Ndungu; Jessica Brosnahan; Thomas P Monath; Barbara Lemaître; Stéphane Grillet; Miriam Botto; Olivier Engler; Jasmine Portmann; Denise Siegrist; Philip Bejon; Peter Silvera; Peter Kremsner; Claire-Anne Siegrist
External link
Publication Year
Publication Journal
Associeted Project
Microbiology or Immunology
Lista de serviços
-
Systems vaccinology: its promise and challenge for HIV vaccine development.Systems vaccinology: its promise and challenge for HIV vaccine development.
-
Systems vaccinology: learning to compute the behavior of vaccine induced immunity.Systems vaccinology: learning to compute the behavior of vaccine induced immunity.
-
Systems biology of vaccination in the elderly.Systems biology of vaccination in the elderly.
-
Immunity to viruses: learning from successful human vaccines.Immunity to viruses: learning from successful human vaccines.
-
Systems biological approaches to measure and understand vaccine immunity in humans.Systems biological approaches to measure and understand vaccine immunity in humans.
-
Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response.Gene signatures related to B-cell proliferation predict influenza vaccine-induced antibody response.
-
Vaccinology in the era of high-throughput biology.Vaccinology in the era of high-throughput biology.
-
Systems vaccinology: Enabling rational vaccine design with systems biological approaches.Systems vaccinology: Enabling rational vaccine design with systems biological approaches.
-
Systems Vaccinology Applied to DNA Vaccines: Perspective and Challenges.Systems Vaccinology Applied to DNA Vaccines: Perspective and Challenges.
-
Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.Adjuvanting a Simian Immunodeficiency Virus Vaccine with Toll-Like Receptor Ligands Encapsulated in Nanoparticles Induces Persistent Antibody Responses and Enhanced Protection in TRIM5α Restrictive Macaques.
-
Methods for predicting vaccine immunogenicity and reactogenicity.Methods for predicting vaccine immunogenicity and reactogenicity.
-
Antigenicity prediction and vaccine recommendation of human influenza virus A (H3N2) using convolutional neural networks.Antigenicity prediction and vaccine recommendation of human influenza virus A (H3N2) using convolutional neural networks.
-
Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine.Pneumococcal colonization impairs mucosal immune responses to live attenuated influenza vaccine.
-
Human Transcriptomic Response to the VSV-Vectored Ebola Vaccine.Human Transcriptomic Response to the VSV-Vectored Ebola Vaccine.
-
Induction of Cell Cycle and NK Cell Responses by Live-Attenuated Oral Vaccines against Typhoid Fever.Induction of Cell Cycle and NK Cell Responses by Live-Attenuated Oral Vaccines against Typhoid Fever.
-
Systems Biology Analysis of the Radiation-Attenuated Schistosome Vaccine Reveals a Role for Growth Factors in Protection and Hemostasis Inhibition in Parasite Survival.Systems Biology Analysis of the Radiation-Attenuated Schistosome Vaccine Reveals a Role for Growth Factors in Protection and Hemostasis Inhibition in Parasite Survival.
-
Molecular alterations in human milk in simulated maternal nasal mucosal infection with live attenuated influenza vaccinationMolecular alterations in human milk in simulated maternal nasal mucosal infection with live attenuated influenza vaccination
-
Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood.Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood.
-
Systems analysis of protective immune responses to RTS,S malaria vaccination in humans.Systems analysis of protective immune responses to RTS,S malaria vaccination in humans.
-
Systems vaccinology.Systems vaccinology.
-
Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans.Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans.
-
Systems biology of vaccination for seasonal influenza in humans.Systems biology of vaccination for seasonal influenza in humans.
-
Prior upregulation of interferon pathways in the nasopharynx impacts viral shedding following live attenuated influenza vaccine challenge in childrenPrior upregulation of interferon pathways in the nasopharynx impacts viral shedding following live attenuated influenza vaccine challenge in children
-
Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures.Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures.
-
Molecular signatures of antibody responses derived from a systems biology study of five human vaccines.Molecular signatures of antibody responses derived from a systems biology study of five human vaccines.
-
Long noncoding RNAs are involved in multiple immunological pathways in response to vaccination.Long noncoding RNAs are involved in multiple immunological pathways in response to vaccination.
-
Metabolic Phenotypes of Response to Vaccination in Humans.Metabolic Phenotypes of Response to Vaccination in Humans.
-
Hidden in plain sight: uncovering the role of CREB1 in HIV-1 vaccine-induced immunityHidden in plain sight: uncovering the role of CREB1 in HIV-1 vaccine-induced immunity
-
Transcriptomic signatures induced by the Ebola virus vaccine rVSV-ZEBOV-GP in adult cohorts in Europe, Africa, and North America: a molecular biomarker studyTranscriptomic signatures induced by the Ebola virus vaccine rVSV-ZEBOV-GP in adult cohorts in Europe, Africa, and North America: a molecular biomarker study
-
Baseline gene signatures of reactogenicity to Ebola vaccination: a machine learning approach across multiple cohortsBaseline gene signatures of reactogenicity to Ebola vaccination: a machine learning approach across multiple cohorts
-
Global blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GPGlobal blood miRNA profiling unravels early signatures of immunogenicity of Ebola vaccine rVSVΔG-ZEBOV-GP
-
COVID-19 vaccination atlas using an integrative systems vaccinology approach.COVID-19 vaccination atlas using an integrative systems vaccinology approach.
-
Emulsion adjuvant-induced uric acid release modulates optimal immunogenicity by targeting dendritic cells and B cells.Emulsion adjuvant-induced uric acid release modulates optimal immunogenicity by targeting dendritic cells and B cells.
-
System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans.System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans.
-
Poly I:C elicits broader and stronger humoral and cellular responses to a Plasmodium vivax circumsporozoite protein malaria vaccine than Alhydrogel in mice.Poly I:C elicits broader and stronger humoral and cellular responses to a Plasmodium vivax circumsporozoite protein malaria vaccine than Alhydrogel in mice.