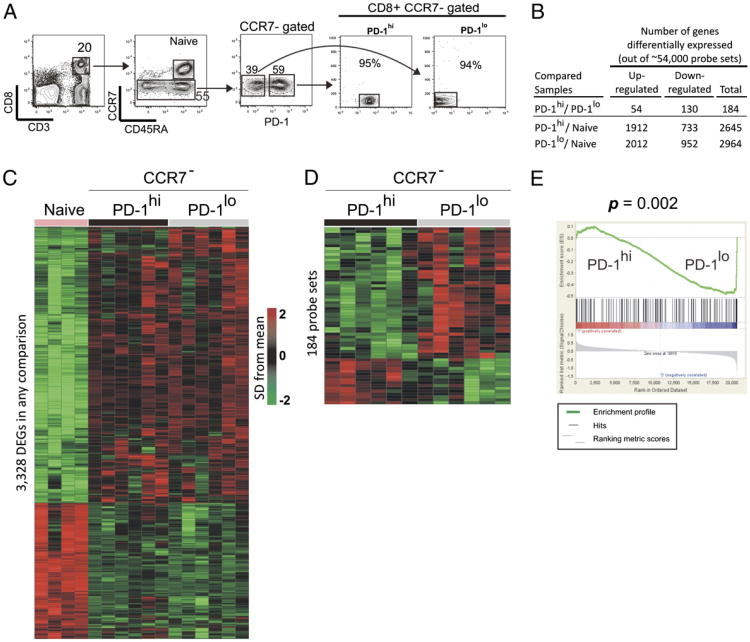
Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults.
T cell dysfunction is an important feature of many chronic viral infections. In particular, it was shown that programmed death-1 (PD-1) regulates T cell dysfunction during chronic lymphocytic choriomeningitis virus infection in mice, and PD-1(hi) cells exhibit an intense exhausted gene signature. These findings were extended to human chronic infections such as HIV, hepatitis C virus, and hepatitis B virus. However, it is not known if PD-1(hi) cells of healthy humans have the traits of exhausted cells. In this study, we provide a comprehensive description of phenotype, function, and gene expression profiles of PD-1(hi) versus PD-1(lo) CD8 T cells in the peripheral blood of healthy human adults as follows: 1) the percentage of naive and memory CD8 T cells varied widely in the peripheral blood cells of healthy humans, and PD-1 was expressed by the memory CD8 T cells; 2) PD-1(hi) CD8 T cells in healthy humans did not significantly correlate with the PD-1(hi) exhausted gene signature of HIV-specific human CD8 T cells or chronic lymphocytic choriomeningitis virus-specific CD8 T cells from mice; 3) PD-1 expression did not directly affect the ability of CD8 T cells to secrete cytokines in healthy adults; 4) PD-1 was expressed by the effector memory compared with terminally differentiated effector CD8 T cells; and 5) finally, an interesting inverse relationship between CD45RA and PD-1 expression was observed. In conclusion, our study shows that most PD-1(hi) CD8 T cells in healthy adult humans are effector memory cells rather than exhausted cells.
Authors
Jaikumar Duraiswamy; Chris C Ibegbu; David Masopust; Joseph D Miller; Koichi Araki; Gregory H Doho; Pramila Tata; Satish Gupta; Michael J Zilliox; Helder I Nakaya; Bali Pulendran; W Nicholas Haining; Gordon J Freeman; Rafi Ahmed
External link
Publication Year
Publication Journal
Associeted Project
Microbiology or Immunology
Lista de serviços
-
As antisense RNA gets intronic.As antisense RNA gets intronic.
-
Androgen responsive intronic non-coding RNAs.Androgen responsive intronic non-coding RNAs.
-
Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.
-
The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.
-
Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.
-
Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.
-
Long non-coding RNAs associated with infection and vaccine-induced immunityLong non-coding RNAs associated with infection and vaccine-induced immunity
-
Comparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophagesComparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophages
-
SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.