The global evolution and impact of systems biology and artificial intelligence in stem cell research and therapeutics development: a scoping review.
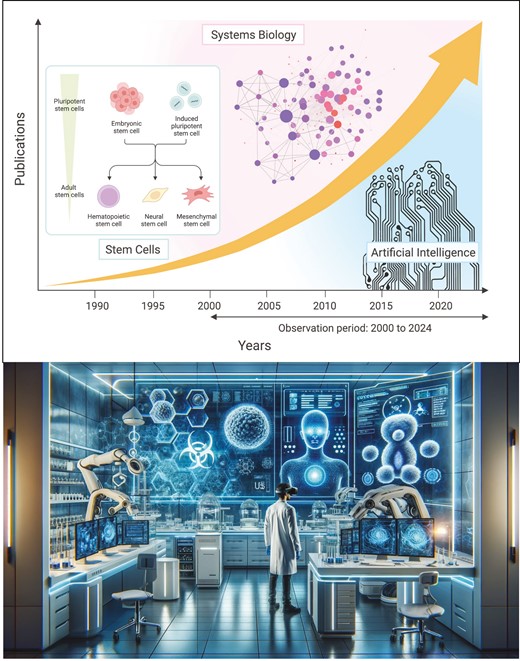
Advanced bioinformatics analysis, such as systems biology (SysBio) and artificial intelligence (AI) approaches, including machine learning (ML) and deep learning (DL), is increasingly present in stem cell (SC) research. An approximate timeline on these developments and their global impact is still lacking. We conducted a scoping review on the contribution of SysBio and AI analysis to SC research and therapy development based on literature published in PubMed between 2000 and 2024. We identified an 8 to 10-fold increase in research output related to all 3 search terms between 2000 and 2021, with a 10-fold increase in AI-related production since 2010. Use of SysBio and AI still predominates in preclinical basic research with increasing use in clinically oriented translational medicine since 2010. SysBio- and AI-related research was found all over the globe, with SysBio output led by the (US, n = 1487), (UK, n = 1094), Germany (n = 355), The Netherlands (n = 339), Russia (n = 215), and France (n = 149), while for AI-related research the US (n = 853) and UK (n = 258) take a strong lead, followed by Switzerland (n = 69), The Netherlands (n = 37), and Germany (n = 19). The US and UK are most active in SCs publications related to AI/ML and AI/DL. The prominent use of SysBio in ESC research was recently overtaken by prominent use of AI in iPSC and MSC research. This study reveals the global evolution and growing intersection among AI, SysBio, and SC research over the past 2 decades, with substantial growth in all 3 fields and exponential increases in AI-related research in the past decade.
Authors
Silva-Sousa T, Usuda JN, Al-Arawe N, Frias F, Hinterseher I
External link
Publication Year
Publication Journal
Associeted Project
Systems Immunology of Human Diseases
Lista de serviços
-
As antisense RNA gets intronic.As antisense RNA gets intronic.
-
Androgen responsive intronic non-coding RNAs.Androgen responsive intronic non-coding RNAs.
-
Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.Conserved tissue expression signatures of intronic noncoding RNAs transcribed from human and mouse loci.
-
The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation.
-
Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer.
-
Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.Insight Into the Long Noncoding RNA and mRNA Coexpression Profile in the Human Blood Transcriptome Upon Leishmania infantum Infection.
-
Long non-coding RNAs associated with infection and vaccine-induced immunityLong non-coding RNAs associated with infection and vaccine-induced immunity
-
Comparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophagesComparative transcriptomic analysis of long noncoding RNAs in Leishmania-infected human macrophages
-
SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.SARS-CoV-2 Selectively Induces the Expression of Unproductive Splicing Isoforms of Interferon, Class I MHC, and Splicing Machinery Genes.